A2L Item 090
- Description: Identify the correct assertion regarding the change of temperature in an expanding gas.
- Goal: Reasoning about adiabatic expansion.
- Source: UMPERG
- Keywords: Ideal Gas, Temperature, Thermodynamics
The question for students:
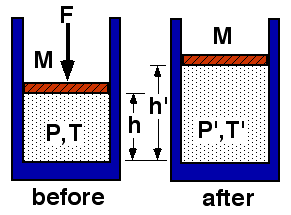
An ideal gas is allowed to expand slowly. The system is thermally isolated.
Which statement regarding the final temperature is true?
- T’ < T
- T’ = T
- T’ > T
- Not enough information
Commentary for teachers:
Answer
(1) For adiabatic expansion, TV(γ-1) is constant. Since the volume increases, the temperature must decrease.
This result can be reasoned by considering the fact that work is done by the gas. Since there is no heat transfer, the internal energy must decrease. Since the internal energy of a perfect gas depends only upon temperature, the temperature must decrease.