A2L Item 110
- Description: Identify the final temperature of two bodies placed in thermal contact.
- Goal: Hone the concept of heat capacity
- Source: UMPERG-ctqpe210
- Keywords: Heat, Thermodynamics
The question for students:
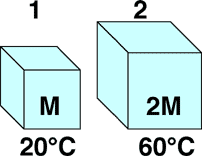
Two thermodynamic systems are made from the same material. The specific heat of this material is independent of temperature. The bodies have different masses and, initially, different temperatures as shown. If the bodies are placed in thermal contact the final equilibrium temperature is most nearly
- 27 C
- 33 C
- 40 C
- 47 C
- None of the above
- Cannot be determined
Commentary for teachers:
Answer
(4) It is valuable to ask students how they obtained their answer. Each of the offered answers is obtained by a common conceptual or algebraic mistake.