A2L Item 190
- Description: Identify the work done on a gas during a thermodynamic cycle.
- Goal: Hone the concept of work for a thermodynamic system
- Source: UMPERG-ctqpe190
- Keywords: Graphing, Ideal Gas, Thermodynamics, Work
The question for students:
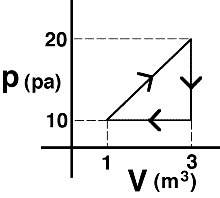 An
ideal gas is taken around the process shown. The net work done
on the gas is most nearly…
An
ideal gas is taken around the process shown. The net work done
on the gas is most nearly…
- 20 J
- -30 J
- 15 J
- -10 J
- none of the above
- cannot be determined
Commentary for teachers:
Answer
(4) The work done ON the system is the negative of the area of the triangle. Students selecting answer #1 or #3 need to be sensitized to the difference between work done on the gas versus by the gas.