A2L Item 195
- Description: Compare the heat for two different thermodynamic paths between two temperatures.
- Goal: Reasoning with thermodynamics
- Source: ctqpe214
- Keywords: Graphing, Thermodynamics
The question for students:
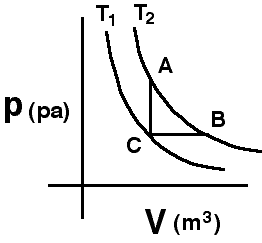 A
system consisting of a quantity of ideal gas has the two isotherms
shown. The system, initially at state C, can be taken along path CA to
final state A or along path CB to state B.
A
system consisting of a quantity of ideal gas has the two isotherms
shown. The system, initially at state C, can be taken along path CA to
final state A or along path CB to state B.
Which of the following is true:
- QCA < QCB
- QCA = QCB
- QCA > QCB
- Not enough information
Commentary for teachers:
Answer
(1) Since the internal energy of an ideal gas depends only on temperature, states A and B have the same internal energy. Along path CB the system does work requiring more heat to be added than along path CA.